Smoke without a fire under a virtual microscope with 100,000,000x magnification
This is about an old chemical trick regarding how to move smoke from one corner of a room to another. We’ll unveil the secret and explain the chemistry reaction behind this trick on the molecular level using a virtual microscope with 100,000,000x magnification. And finally our thoughts as to why we have chosen this chemistry experiment for our first video.
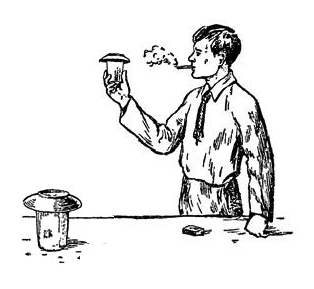
There is an old chemical trick: blow cigarette smoke into a glass and turn it upside down. At the same time, turn another glass over in the opposite corner of the room. The smoke will travel from the first glass across the room and appear in the second one. I tried doing it this weekend and spent a good half an hour coughing my lungs out after a single draw of a cigarette. How on earth do people smoke? ;)
So what’s the secret behind this trick?
Let’s cut to the chase and explain this trick right away. The smoke does not magically travel anywhere. It’s just that while the cigarette smoke settles down in the first glass, new smoke forms in the other glass. There are two gases here – hydrogen chloride (the gas that, when dissolved in water, produces hydrochloric acid) and ammonia (that’s what homeless people smell of). When mixed these two gases produce a solid material called ammonia chloride, whose particles we see as smoke. As you may have guessed, this is exactly what happened in the second glass: the interior of the glass was covered with a concentrated water solution of hydrogen chloride (hydrochloric acid), and the table that the glass was put on was sprayed with a concentrated solution of ammonia. When the glass was put onto the table, the vapors of the two gases started to mix and generate smoke.

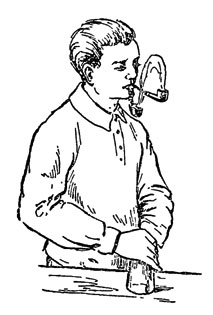
And that’s the trick. There are many ways to use the smoke effect produced by these two liquids when they are brought close enough to each other. Starting from the simplest: take two cotton buds, soak each in a corresponding liquid and enjoy the smoke generation effect by bringing them close together. Or you can take two smoking pipes and impress the public by demonstrating the flow of smoke from one pipe to the other.
But why have we chosen this experiment for our first video?
Initially, I foolishly assumed that we should start with hydrogen burning. I thought that it was the simplest chemical reaction: what can be more primitive than the compound of oxygen and the most basic element - hydrogen? How silly and naïve I was! My reasoning was: a molecule of O2 approaches an H2 molecule, they collide, and if the collision speed is higher than a particular threshold (i.e. the gas is hot enough), the atoms recombine in the most energy-efficient way to form a molecule of H2O. However, this reaction turned out to be not as simple as it looked initially. Russian chemist Nikolay Semenov and English physical chemist Cyril Norman Hinshelwood were jointly awarded the Nobel Prize in Chemistry in 1956 for their researches in the mechanism of this chemical reactions.
Then, I started to consider reactions in solutions producing precipitation. Water-based reactions were also tricky. Ions in water move not as free particles, but rather as complex structures comprising an ion and the water molecules strongly attached to it, the so-called solvate shell. This fact alone shows that reactions in solutions are not as primitive as they seem. I am not talking about complex reactions on the phase boundaries, especially with a catalyst like this or this reaction.
We wanted to start with something simple, and so we went through many chemical reactions and picked this one. We thought that smoke generation during a reaction of hydrogen chloride and ammonia was one of the most easy to understand and visualize chemical reactions.
Our first video
There are many good videos about microbiology. Videos that show processes from the inside. Here are several examples: mRNA translation, DNA replication, from DNA to protein, how DNA is packaged.
However, there are almost no such videos about inorganic chemistry. I mean not videos that just show a beautiful experiment but also show what is happening inside on the molecular level. Now, there is at least one such video :) I hope there will be more soon.
Therefore, let’s imagine that we have a microscope allowing for 100,000,000x magnification. This is the kind of magnification we need to see separate atoms. So we take this microscope and take a closer look:
This was the first time that I was conducting experiments with my children on a weekend and managed to show them what was actually going on inside. This helps you to understand chemistry better than any formulas. Subscribe to our updates and follow us on Twitter to learn about new chemical experiments under our virtual microscope.
Bonus problem
And here comes our bonus problem today: find mistakes in this video. We have made several ones here – some for artistic reasons, and some – due to the lack of time. Can you find them? Tweet us your answers!
See also

CASE STUDY - 8th Grade students at St Timothy's Catholic School use MEL Chemistry to enhance their science lessons
Saint Timothy Catholic School in Mesa is committed to promoting academic excellence in each child it looks after. They encourage self-discipline, self-respect, and respect for others. They understand the importance of engaging students in a comprehensive and relevant curriculum. As a result, the middle school science teacher from St. Timothy Catholic School is using MEL Chemistry subscriptions to enhance and expand their range of learning activities.

CASE STUDY - MEL Chemistry allowing pupils to reach their full potential
The Empower Learning Center is the Alternative Learning Program (ALP) within the Hinckley-Finlayson School District. They offer non-traditional education options for students ages 16-21 in their daytime program, night school for traditional high school students who need to make up credits, and night school for adults 18 and older who would like to complete their diploma or equivalency.
The school was seeking engaging, hands-on chemistry kits to make their science classes more interactive, and to help their students understand key science concepts and achieve their full potential in chemistry.

CASE STUDY - MEL Chemistry at Lund International School, Sweden
Emma Taylor, a science teacher at Lund International School (Sweden), has chosen MEL Chemistry sets as the best option for her students’ science classes. In Lund International School, all programmes are taught in English, and having chemistry sets in English are a great asset to accompany science classes.
Here, Emma shares her experience of how MEL Chemistry sets improved her students’ comprehension and understanding of science concepts.