Why sinks do not rust
If something made of steel and iron is continuously exposed to water, it will rust. Even if the steel is stainless, it will sooner or later corrode as it happens with old ships. Nevertheless, the problem of sink corrosion in our homes is rare. Do you know why?

{Stan in FL: Rust & Ruin in Roatan/ CC BY-NC-SA 2.0}
First of all, what is corrosion?
Everybody has seen rusted metal more than once in a lifetime. What processes make iron look so ugly and cause construction damages? Iron is quite a reactive metal. Being placed in water, it reacts with water and oxygen dissolved in it. This process is called oxidation and leads to a substance of red-and-brown color, also known as rust. Did you know that the color of Mars is also caused by the oxidized iron?

{Huntster: The planet Mars/ CC0}
The principle of metal coating
In order to protect steel and iron from rusting, people cover these materials with other metals that act as heroes sacrificing themselves to merciless water. The best candidates for coating are tin and zinc. Unlike iron, when oxidized, these metals do not leave anything insoluble in water and bad-looking such as rust. In addition, their high abundance and, thereby, low price make them more suitable for coating than other metals.

{Paulnasca: A rusty screw/ CC-BY-2.0}
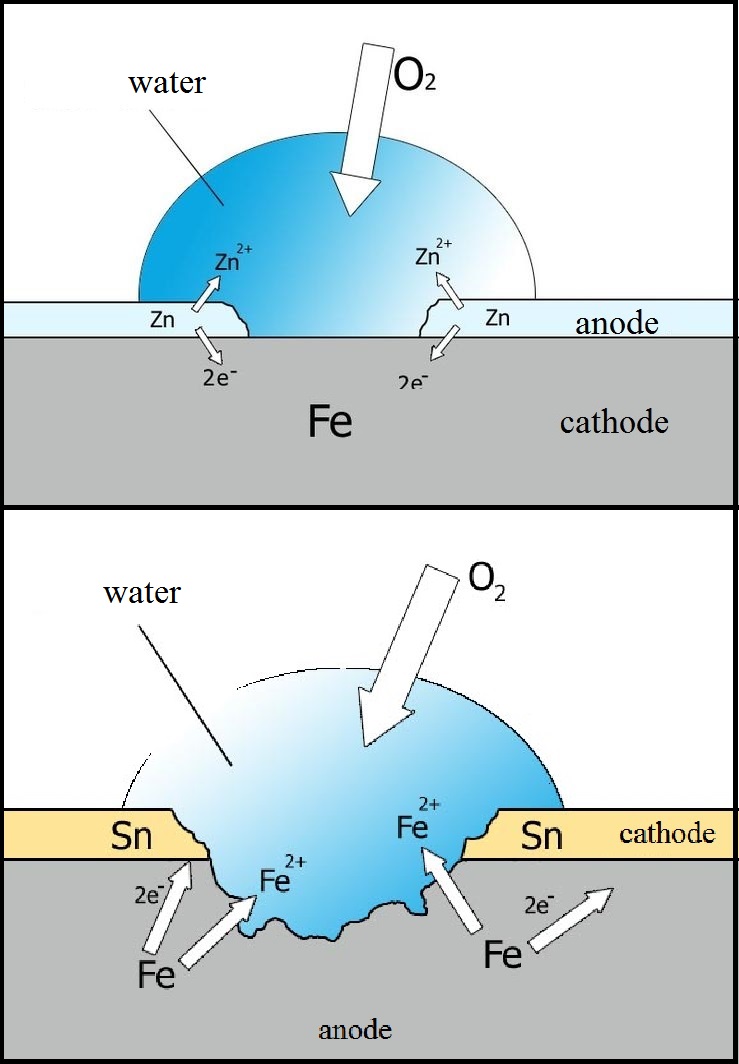
Even if there is a scratch or a little hole in zinc coating, rust won’t touch steel. This happens because zinc feels more comfortable in its compound rather than in the free metal state, and therefore it is much easier to oxidize zinc than iron. In the simultaneous presence of zinc and iron, the latter won’t suffer at all, and zinc corrodes much slower than iron.
{Duk: Zinc chromate conversion coating on small steel parts/ CC BY-SA 3.0}
Unlike zinc, tin is not so reliable. Once the tin coating is damaged, the rust quickly spreads because iron is much more reactive than tin. Nevertheless, if the tin layer is not damaged, iron is safe. Tin slowly reacts with water, and if a tin-covered sink is not intensively scratched by forks and knives rust will never touch it. That is why the Tin Man from “The Wizard of Oz” looks more like the Iron Man: he was so prone to rusting!
How to make a metal coating?
This process is called soldering (or galvanization); and there are two types. The first one is thermal: the coating metal should be melted and the material to be coated (iron, steel) should be put in the melt. This method is not the best – the layer of the coating is not equally thick and micro-sized pores cannot be avoided as they are not avoided when we paint a wall.
The second method is electrochemical. The material to be coated should be put in solution of zinc or tin salt and connected to the negative pole, thus, acting as a cathode. Another conductive material should serve as its positive counterpart (anode) and should also be immersed in the solution. As the current flows, the positively charged zinc/tin ions in solution move to the negatively charged electrode and discharge, transforming into metal atoms and evenly covering the material of the cathode. This process is called electrolysis.
Often, sinks we have in our homes are coated with either tin or zinc. This is the reason why sinks don’t rust.
See also

CASE STUDY - 8th Grade students at St Timothy's Catholic School use MEL Chemistry to enhance their science lessons
Saint Timothy Catholic School in Mesa is committed to promoting academic excellence in each child it looks after. They encourage self-discipline, self-respect, and respect for others. They understand the importance of engaging students in a comprehensive and relevant curriculum. As a result, the middle school science teacher from St. Timothy Catholic School is using MEL Chemistry subscriptions to enhance and expand their range of learning activities.

CASE STUDY - MEL Chemistry allowing pupils to reach their full potential
The Empower Learning Center is the Alternative Learning Program (ALP) within the Hinckley-Finlayson School District. They offer non-traditional education options for students ages 16-21 in their daytime program, night school for traditional high school students who need to make up credits, and night school for adults 18 and older who would like to complete their diploma or equivalency.
The school was seeking engaging, hands-on chemistry kits to make their science classes more interactive, and to help their students understand key science concepts and achieve their full potential in chemistry.

CASE STUDY - MEL Chemistry at Lund International School, Sweden
Emma Taylor, a science teacher at Lund International School (Sweden), has chosen MEL Chemistry sets as the best option for her students’ science classes. In Lund International School, all programmes are taught in English, and having chemistry sets in English are a great asset to accompany science classes.
Here, Emma shares her experience of how MEL Chemistry sets improved her students’ comprehension and understanding of science concepts.
