Let us introduce you... metals!
About 12 000 years ago a man found an unknown stone, which melted in fire without splitting up. That stone was a piece of copper. Without exaggeration, that was probably the most important day in human history, and so began the metal era of humanity, which continues up to present date. Metals surround us everywhere, but do we know what they are and why do they have their remarkable properties?

{Graela: Fire Assay/ CC BY-NC-SA 2.0}
##”We need to go deeper…”
There is a golden rule in chemistry: the origin of any object’s property is deep in its structure. Metals prove this rule: their structure is quite unusual. When metal atoms form solid objects, they are positively charged (let us call them ions) and are “glued” together by the negative outer shell electrons freely flowing in between. These electrons no longer belong to any atom - they are mutual. To better visualize it imagine “electron gas”. The system of positive ions bound by mutual electrons is called metallic bonding.
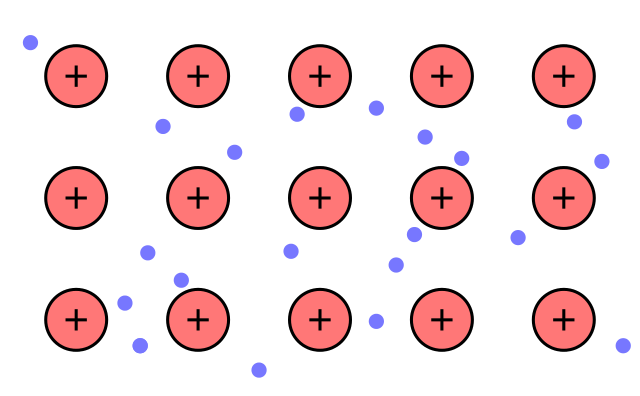
{ARTE: Structure of metal/ CC0}
This short animation does excellent job in illustrating it:
Such a structure explains many properties of metals. So let us take a closer look at some of them.
##Luster
Metals are beautiful, primarily because of their metallic luster, and, thus, have long been used in making jewelry. The “electron gas”, mentioned above, can explain their shiny appearance. Freely moving electrons almost completely reflect falling light, and we see this reflection as a metallic shine. So next time you see a beautiful piece of jewelry – you will know why it glitters!

{Serendipity Diamonds: Golden rings/ CC BY-ND 2.0}
##Remarkable material

{Barry: Forging/ CC BY-NC-SA 2.0}
Most metals are very ductile. Just imagine: one can roll a gold nugget into a 0,003 mm sheet. This is possible without breaking metallic bonds, since during the deformation flowing electrons of the bonds also move. Although the bonds move, they cannot be easily broken, because of the high bond energy. High energy also explains the strength of metals as well as their high melting and boiling points.
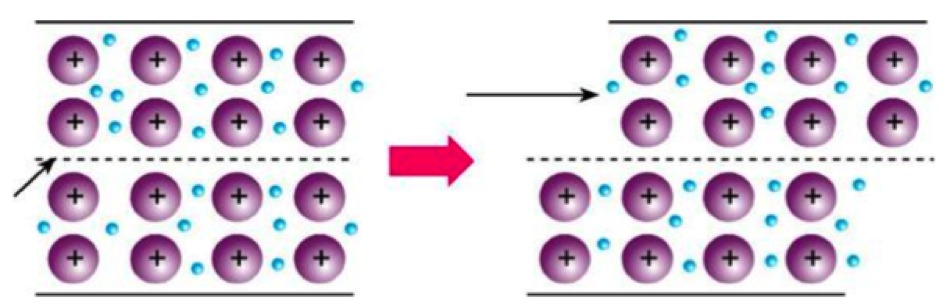
{Deformation of metals}
Ductility and strength make metals a great material for forging – they are malleable. However, the ease of shaping may change at low temperature under which metals become somewhat brittle.
##Delivering current to your home
It is no news that wires are made of metal, because metals conduct electricity. Their conducting property can be explained using metal bonding theory. As we know, metals have mobile electrons shuffling around. So under the influence of even a small difference in potential they can easily move from the negative pole to the positive one, forming electric current.

{arbyreed: Multi-line telephone cables/ CC BY-NC-SA 2.0}
##Are metals magnets?
Everybody knows, that magnets can attract metal objects. Not all of them, though. For example, there will be no magnetic attraction to sodium. Most of metal objects we use in everyday life are made of iron, which is ferromagnetic. It means that below a certain temperature, that is much higher than room temperature, it has a magnetic moment in the absence of an external magnet. This magnetic moment is a reason of the attraction to magnets. Magnetic properties of iron are widely used in many areas.
{Sarnil Prasad: Fridge magnets/ CC BY 2.0}
##Always there for you
Metals dominate periodic table: 94 out of 118 elements ever discovered are metals. Metals even make up at least 3% of our body. (Do not worry, though: airport metal detectors won’t go off if you pass by!) People consume more than 1.5 billion tons of metal annually. We use metals every day and can hardly imagine our life without them. Their remarkable properties make metals essential and irreplaceable.
See also

CASE STUDY - 8th Grade students at St Timothy's Catholic School use MEL Chemistry to enhance their science lessons
Saint Timothy Catholic School in Mesa is committed to promoting academic excellence in each child it looks after. They encourage self-discipline, self-respect, and respect for others. They understand the importance of engaging students in a comprehensive and relevant curriculum. As a result, the middle school science teacher from St. Timothy Catholic School is using MEL Chemistry subscriptions to enhance and expand their range of learning activities.

CASE STUDY - MEL Chemistry allowing pupils to reach their full potential
The Empower Learning Center is the Alternative Learning Program (ALP) within the Hinckley-Finlayson School District. They offer non-traditional education options for students ages 16-21 in their daytime program, night school for traditional high school students who need to make up credits, and night school for adults 18 and older who would like to complete their diploma or equivalency.
The school was seeking engaging, hands-on chemistry kits to make their science classes more interactive, and to help their students understand key science concepts and achieve their full potential in chemistry.

CASE STUDY - MEL Chemistry at Lund International School, Sweden
Emma Taylor, a science teacher at Lund International School (Sweden), has chosen MEL Chemistry sets as the best option for her students’ science classes. In Lund International School, all programmes are taught in English, and having chemistry sets in English are a great asset to accompany science classes.
Here, Emma shares her experience of how MEL Chemistry sets improved her students’ comprehension and understanding of science concepts.