How did scotch tape lead to a Nobel Prize?
The two most commonly known forms of carbon that share the same simplest formula “C” are diamond and graphite. But did you know that there are more, including the one that was discovered using a scotch tape by a scientist who made frogs levitate?

Image by Sergey Slyusarev @ MEL Science / CC BY NC-ND
##How do diamond and graphite differ?
Let’s imagine you want to build a pyramid using a pack of sugar cubes. There are multiple ways to do it and, depending on which one you choose, the resultant pyramids will differ in stability, resistance to wind, and so on.

Image by Doctortc / CC0
The same rule applies to atoms. Arranging atoms of a certain element in multiple different ways will give you the same simplest formula, but different properties, such as boiling and melting points, hardness, density, or chemical reactivity. Below you can see how carbon atoms are arranged in the two structural forms (allotropes) of carbon – graphite and diamond, whose properties differ a lot: diamond is a very hard, transparent material, while grey-colored graphite, which is used to make pencils, can be easily sliced.
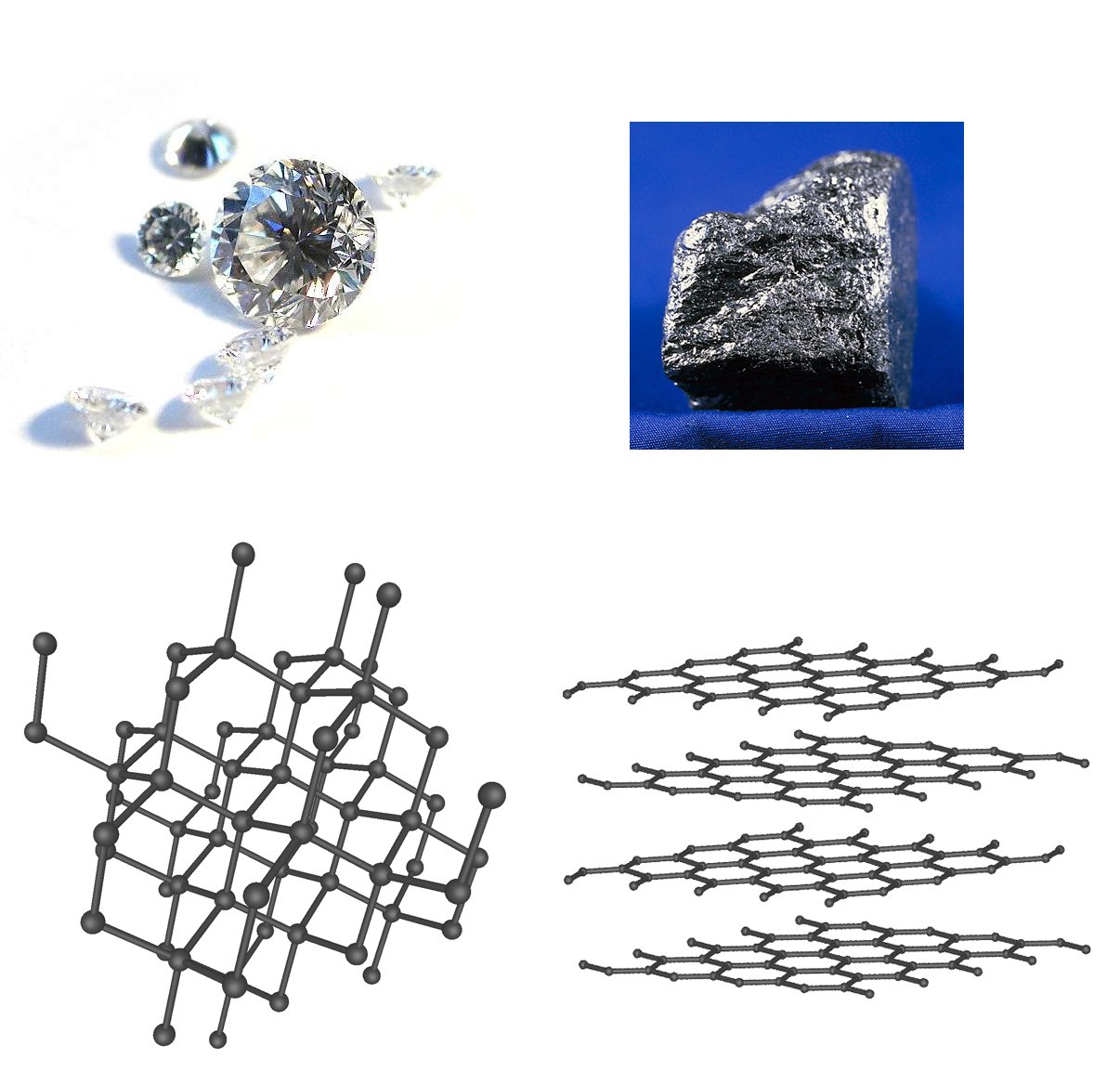
Diamond (left) and graphite (right) by Itub / CC0
The video below explains the difference between diamond and graphite in more detail:
##Is it possible to arrange carbon atoms in any other way?
Diamond and graphite are not the only ways of arranging carbon atoms. In 1985, a group of English scientists discovered an allotrope of carbon named fullerene, which resembles a soccer ball, as you can see from the picture below. Fullerenes differ by the number of carbon atoms in a single ball and have very interesting properties, which makes studying these molecules one of the most promising research directions nowadays.
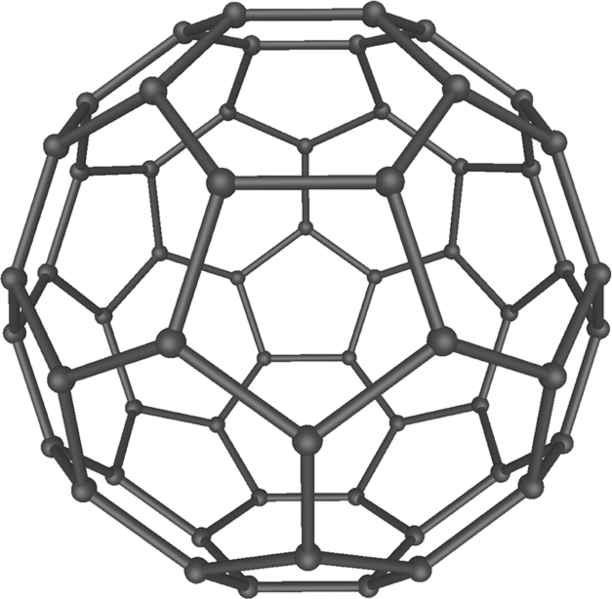
Fullerene C60 by Mstroeck / CC0
There is also another carbon allotrope, which is of great interest in modern science. It is called carbon nanotube, often abbreviated as CNT. Nanotubes differ by the number of atoms in a single section, by the twist angle, and so on. The main application of the CNTs is in nano-sized conductors and semiconductors.
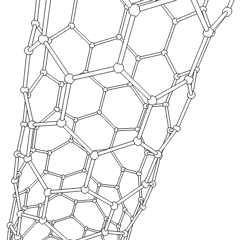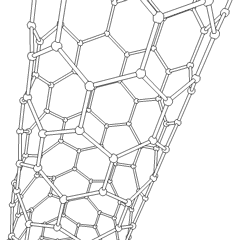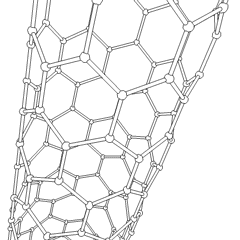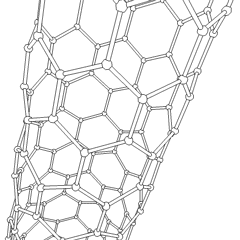
Carbon nanotubes
##The discovery of graphene
The latest carbon allotrope, graphene, was discovered by A. Geim and K. Novoselov from University of Manchester in 2003. It represents a single layer of graphite and has really extraordinary properties, one of which is its exceptional electroconductivity.
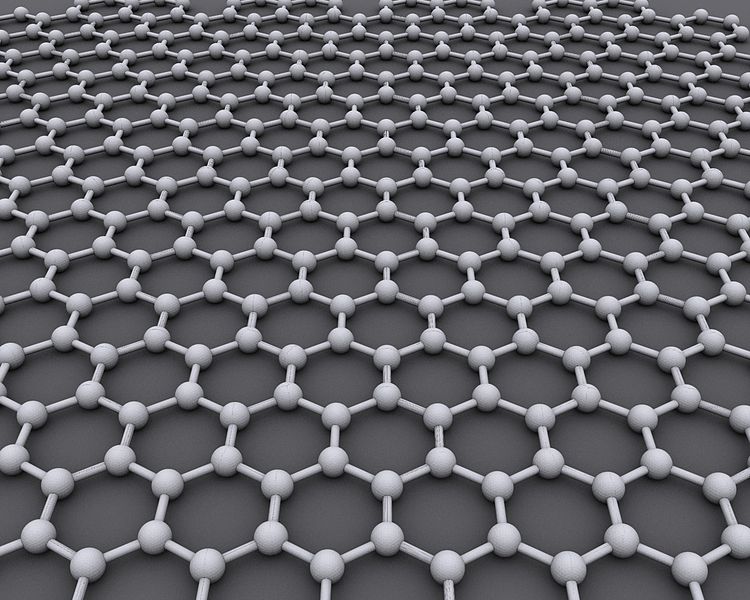
Graphene structure” by AlexanderAIUC / CC0
The story of graphene discovery is actually quite peculiar. The scientists attached a piece of scotch tape to a sample of graphite and then tore it away, separating one layer of atoms from another. After they had done that repeatedly several times, a single layer of graphite, i. e. graphene, was obtained.

Image by Gabriel Hildebrand / CC0
This discovery was awarded a Nobel Prize in 2010. It is also worth mentioning that Andre Geim, one of the discoverers, was awarded an Ignobel Prize ten years earlier for the frog magnet levitation.
Got interested? Here is a video that shows all the above-stated allotropes in 3D:
See also

CASE STUDY - 8th Grade students at St Timothy's Catholic School use MEL Chemistry to enhance their science lessons
Saint Timothy Catholic School in Mesa is committed to promoting academic excellence in each child it looks after. They encourage self-discipline, self-respect, and respect for others. They understand the importance of engaging students in a comprehensive and relevant curriculum. As a result, the middle school science teacher from St. Timothy Catholic School is using MEL Chemistry subscriptions to enhance and expand their range of learning activities.

CASE STUDY - MEL Chemistry allowing pupils to reach their full potential
The Empower Learning Center is the Alternative Learning Program (ALP) within the Hinckley-Finlayson School District. They offer non-traditional education options for students ages 16-21 in their daytime program, night school for traditional high school students who need to make up credits, and night school for adults 18 and older who would like to complete their diploma or equivalency.
The school was seeking engaging, hands-on chemistry kits to make their science classes more interactive, and to help their students understand key science concepts and achieve their full potential in chemistry.

CASE STUDY - MEL Chemistry at Lund International School, Sweden
Emma Taylor, a science teacher at Lund International School (Sweden), has chosen MEL Chemistry sets as the best option for her students’ science classes. In Lund International School, all programmes are taught in English, and having chemistry sets in English are a great asset to accompany science classes.
Here, Emma shares her experience of how MEL Chemistry sets improved her students’ comprehension and understanding of science concepts.