Why do shrimps, crabs and lobsters "blush" when cooked?
Have you ever heard of a unique blue lobster, which is one in a million? Did you know that the pigment responsible for its color is actually …red? It’s that same color that other crustaceans—crabs, shrimps, and the like—turn to when boiled, even though they can otherwise span all possible colors of rainbow (just google “mantis shrimp”!). Where does the red pigment come from, what do crustaceans do with it to look so gorgeously different, and why to they “blush” when boiled?

{by Ruth_W Blue lobster/ CC BY-NC-ND 2.0}
Where does the red pigment come from?
In short, animals don’t synthesize most types of bright colors in their bodies; they borrow them from food. So do shrimps, crabs and lobsters: they take the red pigment from their plant food (red algae). This is a standard practice in the animal kingdom, and crustaceans pass the pigment on to birds and other predators!

{by Evangelio Gonzalez MD: Flamingo / CC BY-NC-ND 2.0}
What is the chemistry of this red pigment?
Astaxanthin – the red pigment, a close relative to the orange carotene – is responsible for all the colors we discuss here: of red algae, crustaceans (shrimps, crabs and lobsters) and birds (flamingo). The reason for the color change is the introduction of 4 oxygen atoms, which lengthen the conjugated system from 11 double bonds in carotene to 13 in astaxanthin.
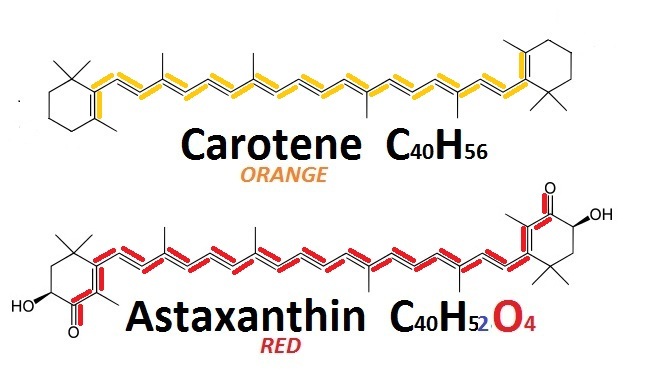
{by anton.miro Structure of carotene and astaxanthin/ CC0}
How is the pigment distributed in the crab shell?
National flags of Ecuador, Columbia and Venezuela have the same arrangement of colors which can help us to demonstrate the specifics of distribution of astaxanthin in crustaceans.

{by anton.miro / CC BY 2.0}
Surprisingly, both blue and yellow—the two additional basic colors of crustaceans—are also created by astaxanthin under different conditions. Normally red (in the skin), it turns either blue (the so-called enolatе form) or yellow when it binds to specific proteins in different layers of the shell.
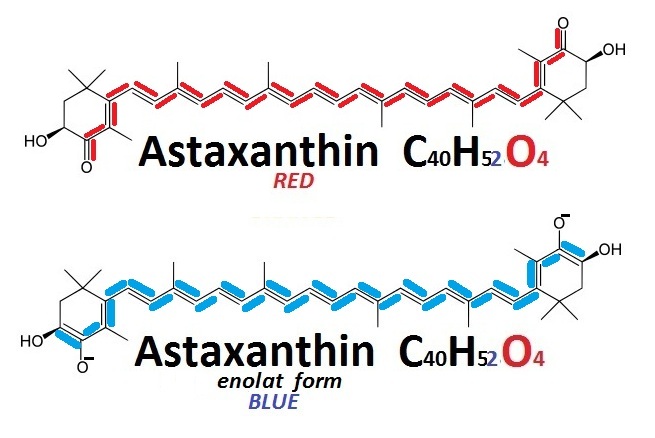
{by anton.miro: Different forms of astaxanthin/ CC BY 2.0}
So how do crustaceans do this multi-color trick and turn from brown, grey, greenish, yellowish, with rarer blue and even more exotic red or calico variations to red when boiled? The colors of the upper layers are semi-transparent, so the resulting color we observe is their superposition. Thus, we end up with any color: from standard grey with green shades (yellow+blue gives green!) to blue and even red (when upper-layer colors are not fully formed) and calico when distribution of the pigment is uneven, resulting in the formation of the spots.

{by Ben Sutherland: / CC BY 2.0}
What happens when we cook crustaceans?
Normally, astaxanthin is thermally stable. It’s not the case, though, for its protein-bound form. Human body, for example, cannot sustain temperatures higher than 40 degrees Celsius for this very reason: proteins lose their structure (and, thus, change their properties) when heated. So after boiling for some time, astaxanthin is released from its protein-bound form and all three layers of crab’s skin and shell turn red.

{by anton.miro / CC0}
Summary
What most people would say about the color of crustaceans is this: 1/ They’re alive and are kind of grey…ish; 2/ They turn red when boiled;
What most of them would miss, though, is that all these colors, including a rare blue of crabs and lobsters, are actually present in our 16-legged friends all the time, provided by one pigment, a relative of an orange carotene – red astaxanthin. Remember this next time you’re enjoying lobster with your friends!

{by postaletrice: Lobster fishing in Brittany (c.1910)/ CC0}
See also

CASE STUDY - 8th Grade students at St Timothy's Catholic School use MEL Chemistry to enhance their science lessons
Saint Timothy Catholic School in Mesa is committed to promoting academic excellence in each child it looks after. They encourage self-discipline, self-respect, and respect for others. They understand the importance of engaging students in a comprehensive and relevant curriculum. As a result, the middle school science teacher from St. Timothy Catholic School is using MEL Chemistry subscriptions to enhance and expand their range of learning activities.

CASE STUDY - MEL Chemistry allowing pupils to reach their full potential
The Empower Learning Center is the Alternative Learning Program (ALP) within the Hinckley-Finlayson School District. They offer non-traditional education options for students ages 16-21 in their daytime program, night school for traditional high school students who need to make up credits, and night school for adults 18 and older who would like to complete their diploma or equivalency.
The school was seeking engaging, hands-on chemistry kits to make their science classes more interactive, and to help their students understand key science concepts and achieve their full potential in chemistry.

CASE STUDY - MEL Chemistry at Lund International School, Sweden
Emma Taylor, a science teacher at Lund International School (Sweden), has chosen MEL Chemistry sets as the best option for her students’ science classes. In Lund International School, all programmes are taught in English, and having chemistry sets in English are a great asset to accompany science classes.
Here, Emma shares her experience of how MEL Chemistry sets improved her students’ comprehension and understanding of science concepts.