Turning Carbone Dioxide into Useful Products
According to the Earth System Research Laboratory, on April 2015 the concentration of carbon dioxide in the atmosphere has reached the critical value of 403.26 parts per million. What’s worse, though, is that this number increases every year. It is not a secret that carbon dioxide, the man-made portion of which mainly comes from burning fossil fuels and land use change, is the main cause of the greenhouse effect. Can we make anything useful from this enormous amount that will benefit humanity?
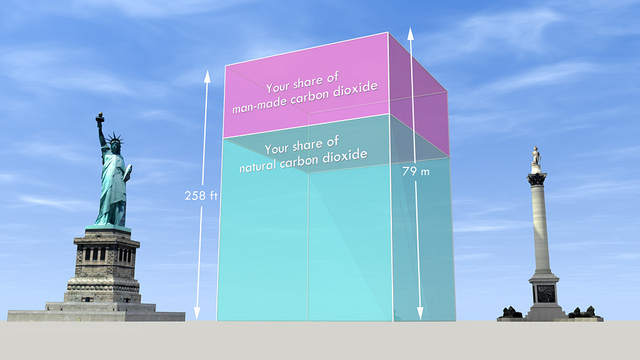
{by Carbon Visuals: Your share of global carbon dioxide/ CC BY 2.0}
A Serious Problem
On May 9th, 2013 the concentration of carbon dioxide (CO2) at the Mauna Loa Observatory in Hawaii had reached 400 parts per million (ppm). Taking into account the fact that before the advent of industrialization the concentration of CO2 was around 280 ppm, that’s a very big number. In fact, until recently the concentration of CO2 has never exceeded 300 ppm.
Here you can learn more about the history of the atmospheric CO2 levels:
Measurement of the CO2 Concentration
The measurements made at the Mauna Loa Observatory have historical significance. There, in 1958 David Keeling invented a method of estimating the quantity of CO2 in the atmosphere by measuring the amount of infrared (IR) light absorbed by this gas in a cylindrical cell pumped with air. The more carbon dioxide was in the cell, the more IR light was absorbed and the less light reached the detector. Below you can see a graph that shows the constant change in the CO2 concentration in the atmosphere since 1958 – the so-called Keeling Curve.
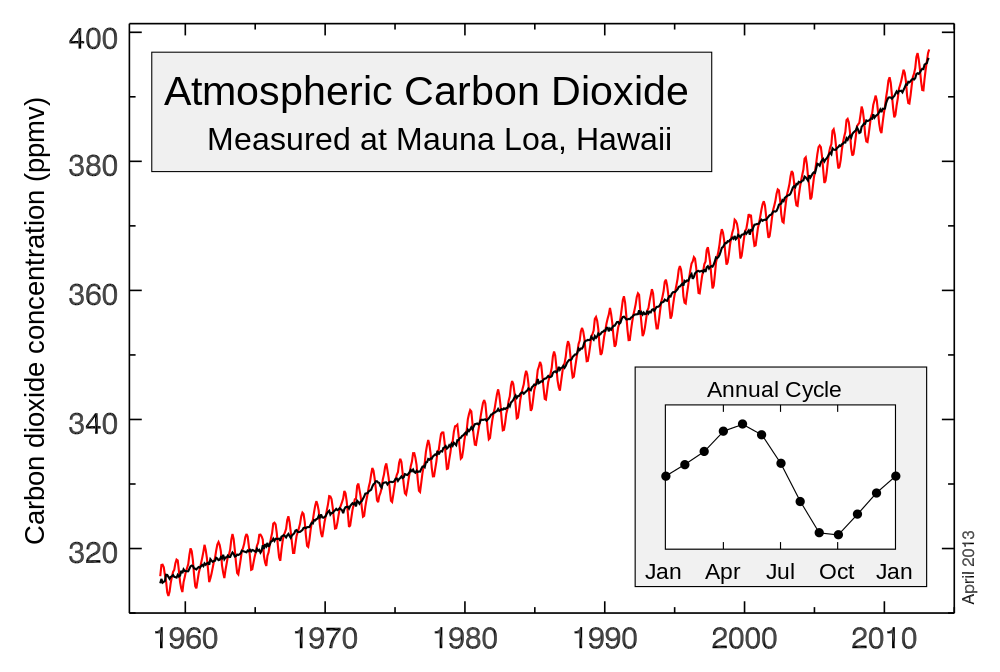
{by Naratanese, Semhur, and the NOAA: Keeling Curve / CC SA-BY 3.0}
Ways of Turning Carbone Dioxide into Something Useful
Carbon dioxide, along with water, is produced when fuels (usually, hydrocarbons) are burned. These combustion processes are accompanied by the release of a great amount of energy. It has always been the scientists’ dream to put some of this energy back into the CO2 molecules to make something useful out of them. The modern technologies of turning carbon dioxide into useful products include harnessing microbes that live in severe environments to make fuels, applying sunlight to form an artificial fuel from hydrogen and carbon monoxide, making methanol in the lab, and reproducing the process of photosynthesis itself. Let’s look closer at some of these technologies.

{by Milan Klusacek / CC BY 2.0}
Making Synthetic Gas from CO2
Recently, the scientists at SOLAR-JET performed a multi-step process of turning carbon dioxide into kerosene that can be used as jet fuel. To do so, they constructed a solar reactor that harnesses the energy of sunlight and can quickly generate temperatures as high as 1500o C. Under these temperatures, metal oxide (e.g. iron, cerium), which is an essential part of the system, reduces to free some oxygen. Then, in the dark side of the reactor, it sucks back the lacking oxygen out of the introduced CO2 and H2O molecules producing carbon monoxide and hydrogen. The mixture of these two gases is called synthetic gas or syngas and is widely used in industry to make hydrocarbons, plastics, and other chemicals. At the final stage of the SOLAR-JET process, syngas is converted to kerosene.
You can learn more about it in this video:

{ by Skyemoor: Example of Solar Reactor/ CC0}
Obstacles Faced by Scientists
Even though much progress has been made by scientists in the field of making useful products from CO2, most of the proposed ideas are considered commercially ineffective and cannot be reproduced at the industrial level. The biggest problem for researchers is sucking carbon dioxide out of the air which is prohibitively expensive. Another big problem is the cost of catalysts used by many research groups to convert CO2 into CO. Most of them use gold and silver to perform this process.
{by OpenClips : Hurdle / CC0}
See also

CASE STUDY - 8th Grade students at St Timothy's Catholic School use MEL Chemistry to enhance their science lessons
Saint Timothy Catholic School in Mesa is committed to promoting academic excellence in each child it looks after. They encourage self-discipline, self-respect, and respect for others. They understand the importance of engaging students in a comprehensive and relevant curriculum. As a result, the middle school science teacher from St. Timothy Catholic School is using MEL Chemistry subscriptions to enhance and expand their range of learning activities.

CASE STUDY - MEL Chemistry allowing pupils to reach their full potential
The Empower Learning Center is the Alternative Learning Program (ALP) within the Hinckley-Finlayson School District. They offer non-traditional education options for students ages 16-21 in their daytime program, night school for traditional high school students who need to make up credits, and night school for adults 18 and older who would like to complete their diploma or equivalency.
The school was seeking engaging, hands-on chemistry kits to make their science classes more interactive, and to help their students understand key science concepts and achieve their full potential in chemistry.

CASE STUDY - MEL Chemistry at Lund International School, Sweden
Emma Taylor, a science teacher at Lund International School (Sweden), has chosen MEL Chemistry sets as the best option for her students’ science classes. In Lund International School, all programmes are taught in English, and having chemistry sets in English are a great asset to accompany science classes.
Here, Emma shares her experience of how MEL Chemistry sets improved her students’ comprehension and understanding of science concepts.