Is Oxygen a Big Deal?
There is a widespread expression: “People say you can’t live without love. I think oxygen is more important.” As for me, I totally agree with the statement. Oxygen is essential for the survival of most organisms on Earth, including ourselves, and if we suffer the lack of this compound, we won’t stay alive for long. However, there are creatures that don’t require oxygen for existence.
{by OpenClips / CC0 }
The History of Oxygen
Long time ago the atmosphere on Earth was completely different from what it is now. It contained a lot of methane, but no oxygen. As a matter of fact, the first oxygen on our planet was produced by cyanobacteria. Initially this gas was captured by iron and organic matter dissolved in ocean water. However, around 2.3 billion years ago, there was so much oxygen that it started to accumulate in the atmosphere. This phenomenon, called Great Oxygenation Event, caused a significant environmental change that led to the extinction of many oxygen-hostile organisms.

{by Matthewjparker: Cyanobacteria/ CC BY-SA 3.0}
Now let’s move closer to our days. In 1772 Swedish scientist Carl Wilhelm Scheele isolated a gas, which he called “fire-air”, because it supported combustion. However, he didn’t publish his work until 1777 and was beaten by another researcher, Joseph Priestley, who announced the discovery of oxygen in 1774. But it wasn’t until 1777, when a scientist named Antoine Lavoisier coined the actual term “oxygen”, which means “acid-producer” in Greek.

{by Tene~commonswiki: Carl Wilhelm Scheele/ CC0 }
Why Do We Need Oxygen?
First and foremost, we need it to breathe, and so do many other organisms. Being an excellent oxidizing agent, it allows organisms to get useful energy to stimulate their activity by breaking down glucose and other food molecules into carbon dioxide (CO2) and water (H2O). This energy is stored in the form of ATP, which stands for adenosine triphosphate — the major energy currency of our cells.
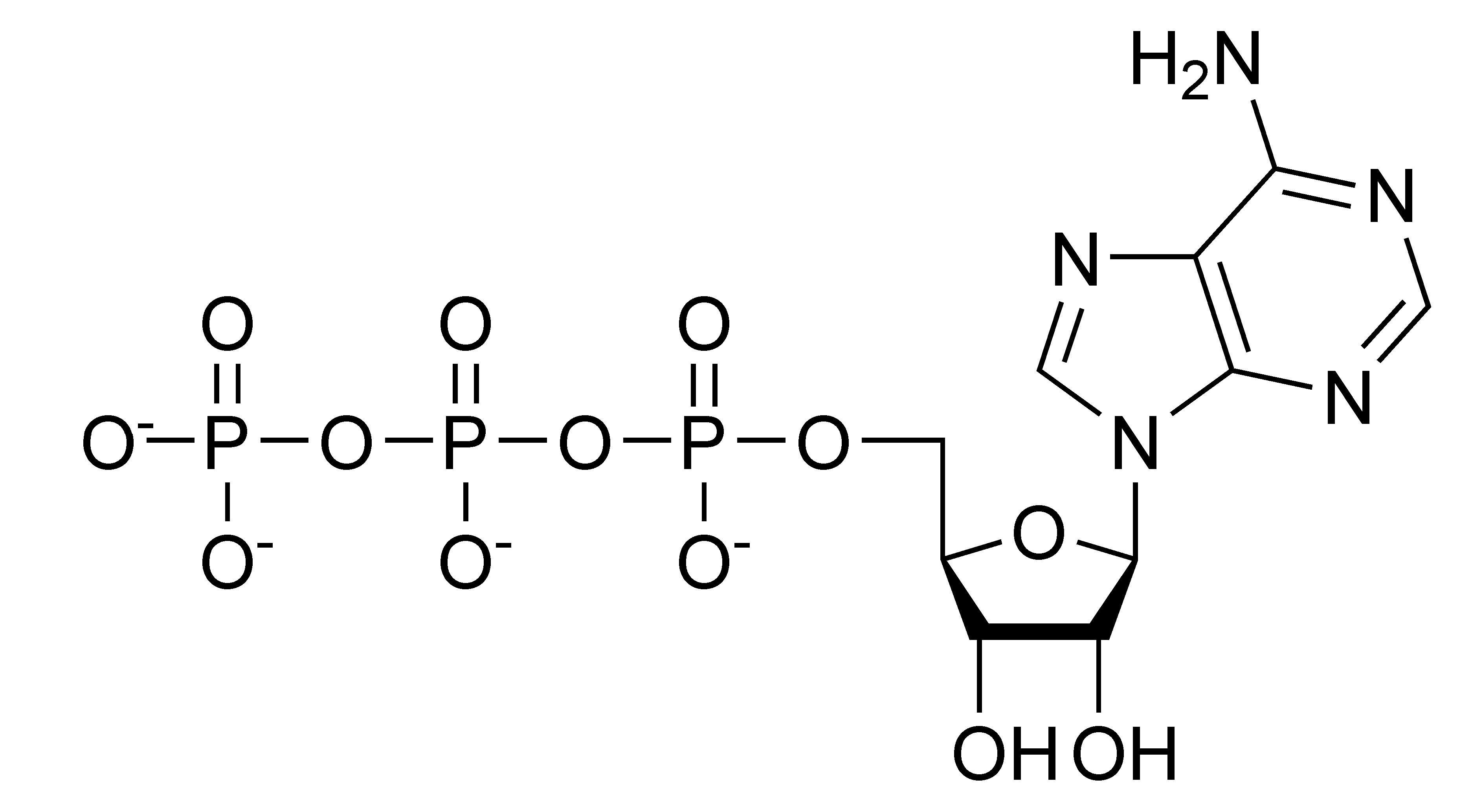
{by Samulili: Structure of ATP/ CC BY-SA 3.0}
The simplified cellular respiration reaction, which takes place in our organism in mitochondria, is written below.
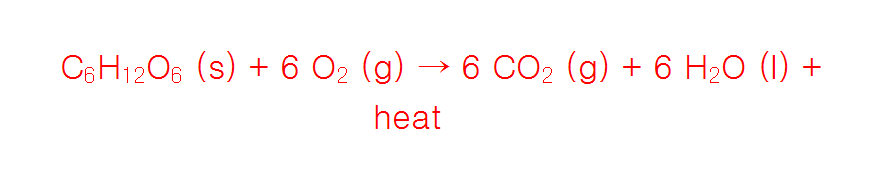
<span style=”color:blue; font-family:Helvetica; font-size:0.7em”{by Anton K @ MEL Science </span> / CC0}
Organisms That Don’t Need Oxygen to survive
Depending on whether or not organisms need oxygen, they can be either aerobic or anaerobic. Anaerobic ones are oxygen-independent and thrive in oxygen-free environments. Interestingly, they sometimes can be found near volcano eruptions and the presence of oxygen causes their death.
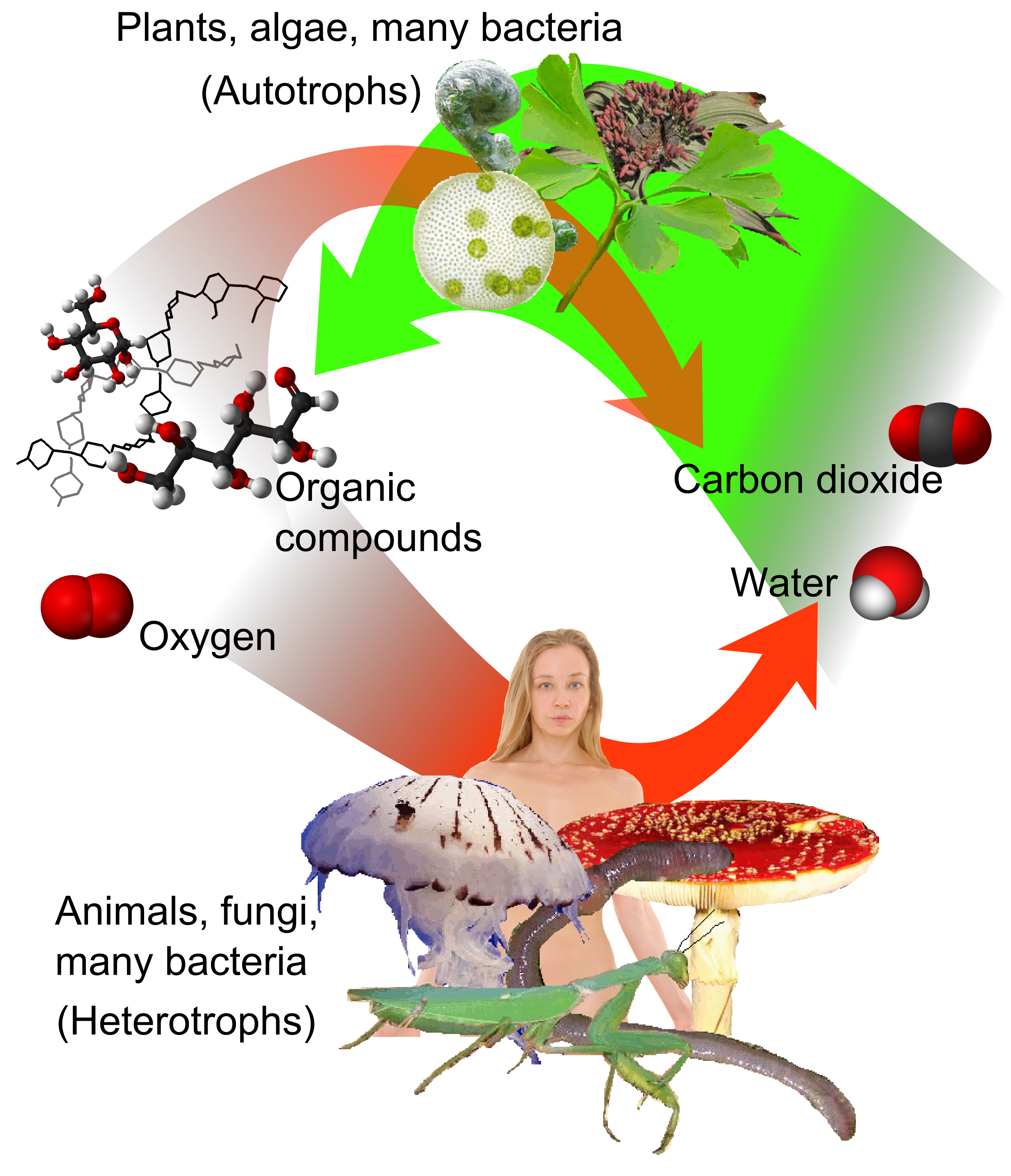
{by Mikael Haggstrom: Aerobic and Anaerobic Respiration/ CC BY-SA 3.0}
You can learn more about anaerobic respiration in this video:
So Is Oxygen Important?
It definitely is necessary for our survival: we are likely to acquire severe and permanent brain damage after not breathing for 5 to 10 minutes. Without oxygen supply to our lungs, our heart will enlarge and our arteries will squeeze, causing blood pressure to rise high enough to kill us. Anaerobes are also dependent on oxygen, actually on its absence, because its presence causes their death. So if they have a chance to acquire communication ability, they will definitely say: “Yes, oxygen is a big deal! That’s why we strive to live where it’s absent.”
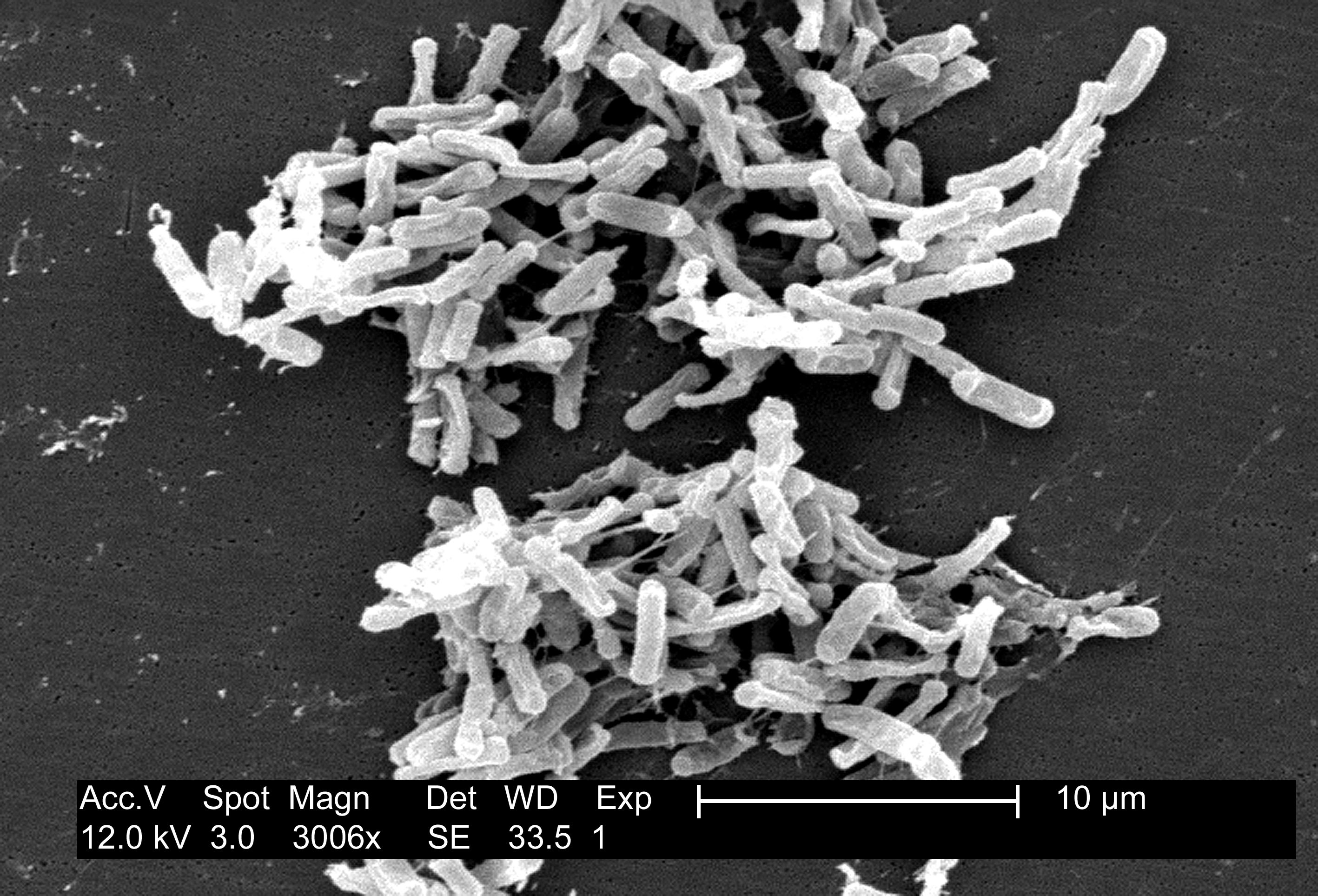
{by Angusmclellan: Clostridium difficile is an example of anaerobic organism / CC0}
See also

CASE STUDY - 8th Grade students at St Timothy's Catholic School use MEL Chemistry to enhance their science lessons
Saint Timothy Catholic School in Mesa is committed to promoting academic excellence in each child it looks after. They encourage self-discipline, self-respect, and respect for others. They understand the importance of engaging students in a comprehensive and relevant curriculum. As a result, the middle school science teacher from St. Timothy Catholic School is using MEL Chemistry subscriptions to enhance and expand their range of learning activities.

CASE STUDY - MEL Chemistry allowing pupils to reach their full potential
The Empower Learning Center is the Alternative Learning Program (ALP) within the Hinckley-Finlayson School District. They offer non-traditional education options for students ages 16-21 in their daytime program, night school for traditional high school students who need to make up credits, and night school for adults 18 and older who would like to complete their diploma or equivalency.
The school was seeking engaging, hands-on chemistry kits to make their science classes more interactive, and to help their students understand key science concepts and achieve their full potential in chemistry.

CASE STUDY - MEL Chemistry at Lund International School, Sweden
Emma Taylor, a science teacher at Lund International School (Sweden), has chosen MEL Chemistry sets as the best option for her students’ science classes. In Lund International School, all programmes are taught in English, and having chemistry sets in English are a great asset to accompany science classes.
Here, Emma shares her experience of how MEL Chemistry sets improved her students’ comprehension and understanding of science concepts.