Alloys on the service of humanity
If you ever watched the X-men series, you know Wolverine – the invulnerable mutant with a skeleton made of metal. He can withstand any damage caused to his body, even a nuclear explosion. His bones are made of adamantium, which is the hardest material ever made. But what makes adamantium so special? Is it real and, if not, are there real-life analogues?

{Max: Wolverine/ CC BY-NC-ND 2.0}
##What is adamantium?
Adamantium is not an individual chemical element - it is a type of alloy. So at first we need to understand what alloys are.

{Andreas Levers: Metal/ CC BY-NC 2.0}
Many years ago, when people first added tin to copper, they invented bronze, which helped them to figure out that adding components to the metal’s melt enhances the metal’s properties. Depending on the type of alloy, some metals become more malleable (e.g. malleable iron), others increase in hardness (e.g. pobedit – an alloy of tungsten carbide with cobalt) or become infusible (e.g. hafnium and tantalum carbides). But why exactly does it happen?

{karstenfotos: Casting Bronze Age Swords/ CC BY-NC 2.0}
Alloys vs. pure metals
Alloys are complex mixtures of either two or more metals (e.g. copper+tin) or a metal and a nonmetal (e.g. iron+carbon), and adding a new component to a pure metal changes its crystal lattice and, thus, alters its properties at the macro level. Some alloys (substitutional) get formed when metal atoms in the crystal lattice are substituted with the atoms of another component of a comparable size. Examples include bronze and brass: copper atoms are replaced by either tin or zinc.
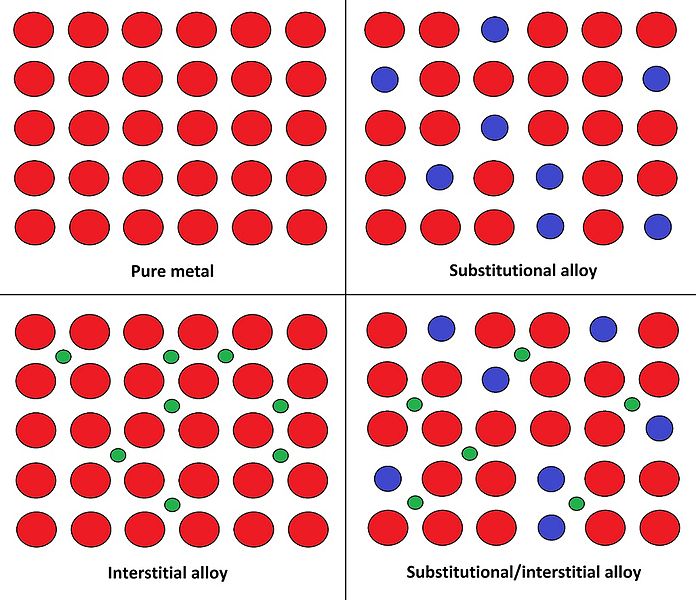
{Zaereth: Atomic arrangements in different types of alloys/ CC0}
(Red circles are metal atoms, green and blue – additives)
Others (interstitial) are formed when atoms get trapped in the spaces between metal atoms. Steel, in which carbon atoms fit into the interstices of the iron matrix, is the most famous example. And, finally, a combination of both types gives us a substitutional/interstitial alloy. Think of stainless steel: nickel and chromium replace some iron atoms, while carbon gets trapped in between.
##Is Wolverine’s secret weapon real?

{Yan Renucci: Wolverine/ CC BY-ND 2.0}
In the Marvel universe, adamantium is an alloy of iron and synthetic resins. In our universe, however, it does not really exist. The whole idea of mixing iron and resins does not seem persuasive for a chemist: resins are not good additives to improve strength; they may only be good for improving ductility.
##Alloys around us

{Bert Kaufmann: Cloud Gate Chicago (Explore)/ CC BY 2.0}
Some alloys are relatively inexpensive and have appropriate properties to be widely used. Steel, which is harder and stronger than iron due to carbon insertions, is the most important construction material. Brass, which is stronger than copper and more resistant to corrosion, is widely used in shipbuilding and the motor industry. Duralumin, which is made by adding copper, magnesium and manganese to aluminum, is hard, but lightweight and is, therefore, a perfect material for the aircraft industry. The list may go on and on.
##Smart alloys
Some alloys have unusual properties. One such example is the shape-memory alloys (e.g. made of nickel and titanium) that can “remember” their original shape and return to it after being heated. This is possible due to the alloy’s thermoplasticity or the ability of the stretched and compressed particles of the deformed matter to relax and return to the original state when thermal energy is applied.

{Ars Electronica: Animated Lamp V/ a. g. r. a./ CC BY-NC-ND 2.0}
(This lamps’ technology is based on shape memory wire. It consists of an alloy that “remembers” its shape and reassumes it as soon as it’s heated up by electrical current)
The whole idea of mixing metals and even nonmetals to make materials with new properties has good prospects in the future. So, do not despair – maybe one day creating adamantium will become possible.
See also

CASE STUDY - 8th Grade students at St Timothy's Catholic School use MEL Chemistry to enhance their science lessons
Saint Timothy Catholic School in Mesa is committed to promoting academic excellence in each child it looks after. They encourage self-discipline, self-respect, and respect for others. They understand the importance of engaging students in a comprehensive and relevant curriculum. As a result, the middle school science teacher from St. Timothy Catholic School is using MEL Chemistry subscriptions to enhance and expand their range of learning activities.

CASE STUDY - MEL Chemistry allowing pupils to reach their full potential
The Empower Learning Center is the Alternative Learning Program (ALP) within the Hinckley-Finlayson School District. They offer non-traditional education options for students ages 16-21 in their daytime program, night school for traditional high school students who need to make up credits, and night school for adults 18 and older who would like to complete their diploma or equivalency.
The school was seeking engaging, hands-on chemistry kits to make their science classes more interactive, and to help their students understand key science concepts and achieve their full potential in chemistry.

CASE STUDY - MEL Chemistry at Lund International School, Sweden
Emma Taylor, a science teacher at Lund International School (Sweden), has chosen MEL Chemistry sets as the best option for her students’ science classes. In Lund International School, all programmes are taught in English, and having chemistry sets in English are a great asset to accompany science classes.
Here, Emma shares her experience of how MEL Chemistry sets improved her students’ comprehension and understanding of science concepts.