Order above all
The famous sinking of Titanic was one of the deadliest peacetime maritime disasters in history. It happened because the liner struck an iceberg in the North Atlantic. There is a strange circumstance, though: a huge ship that could float on the water surface got damaged by an iceberg, which is just a big solid water chunk. This disaster shows how different states of aggregation of the same substance result in their different properties.

{The Tedster: Iceberg Quest Boat Tour/ CC BY-ND 2.0}
#Fundamental states of matter
Every substance can be solid, liquid of gas. The most illustrative example is water, which can take the form of ice, steam or liquid. Metals, for instance, can also be in all these states. If we melt them at high temperature, they become liquid. If the temperature rises further, they become gaseous. Mercury is the only metal, which is liquid at a room temperature. Actually, there is also the fourth state of matter – plasma, which consists of charge carriers: ions and elementary particles. However, plasma is not often found in our life, as it requires extreme conditions.

{Spirit469: Four Fundamental States of Matter/ CC-BY-SA-3.0}
(Clockwise from top left, they are solid, liquid, plasma, and gas, represented by an ice sculpture, a drop of water, electrical arcing from a tesla coil and the air around clouds, respectively).
What is the reason of different states of aggregation? As always, it’s the structure.
##Take a closer look
Solid objects consist of atoms, molecules or ions, which are located in a certain strict order. They form a three-dimensional structure of recurring units, called a crystal lattice. To better visualize a crystal lattice let us try to create an imaginary model. At first, we will make a unit cell – a basic unit, which determines the whole structure of a lattice: for example, one atom A surrounded by four atoms B in a definite order. Then we copy and paste the unit cell many times, just as by copying and pasting texts on your PC. This way a crystal lattice, which is made of many unit cells with atoms located in a certain order, is formed.
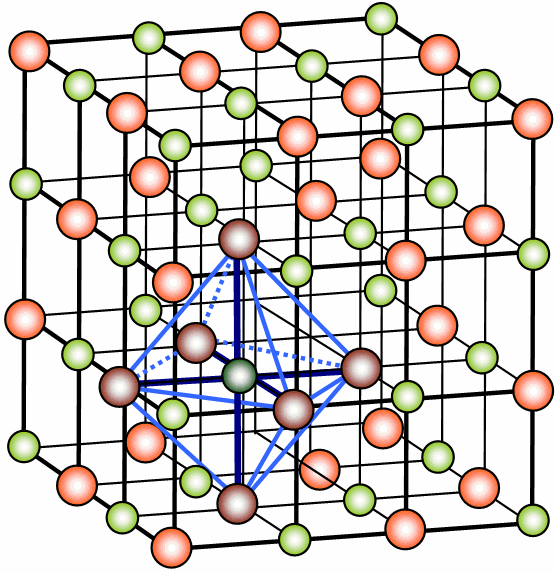
{H. Hoffmeister: NaCl ionic lattice/ CC BY-SA 3.0 }
(The crystal lattice of salt. Green: Na+ ions, red: Cl- ions. The blue figure is a unit cell).
The energy of the particles in a lattice is minimal, as they do not have much freedom to move while being at the lattice sites. The lower the energy of a system, the higher its stability. Thus, we can assume that crystal structure is very stable. Indeed, people use solid objects almost everywhere primarily because of their stability. Liquids and gases do not have an ordered structure, their particles move chaotically, from one place to another.
##Some other types
There are several types of crystal lattices. An atom lattice has atoms in its sites; molecular lattice has molecules; ionic lattice – ions. There is also a metallic crystal lattice: it has positive metal ions in its sites and mutual electrons, which flow between ions like a gas and belong to each one of them. Namely, a metallic lattice determines all characteristic properties of metals.

{O Palsson: The Atomium/ CC BY 2.0}
(The Atomium is a building in Brussels, which represent a unit cell of an iron crystal magnified 165 billion times).
Thus, we see that order plays a significant role in determining any object’s properties. And while a ship like Titanic can freely float on the water surface, the same water molecules in a structured state can form an iceberg, which can be a potential threat to the mentioned ship.
See also

CASE STUDY - 8th Grade students at St Timothy's Catholic School use MEL Chemistry to enhance their science lessons
Saint Timothy Catholic School in Mesa is committed to promoting academic excellence in each child it looks after. They encourage self-discipline, self-respect, and respect for others. They understand the importance of engaging students in a comprehensive and relevant curriculum. As a result, the middle school science teacher from St. Timothy Catholic School is using MEL Chemistry subscriptions to enhance and expand their range of learning activities.

CASE STUDY - MEL Chemistry allowing pupils to reach their full potential
The Empower Learning Center is the Alternative Learning Program (ALP) within the Hinckley-Finlayson School District. They offer non-traditional education options for students ages 16-21 in their daytime program, night school for traditional high school students who need to make up credits, and night school for adults 18 and older who would like to complete their diploma or equivalency.
The school was seeking engaging, hands-on chemistry kits to make their science classes more interactive, and to help their students understand key science concepts and achieve their full potential in chemistry.

CASE STUDY - MEL Chemistry at Lund International School, Sweden
Emma Taylor, a science teacher at Lund International School (Sweden), has chosen MEL Chemistry sets as the best option for her students’ science classes. In Lund International School, all programmes are taught in English, and having chemistry sets in English are a great asset to accompany science classes.
Here, Emma shares her experience of how MEL Chemistry sets improved her students’ comprehension and understanding of science concepts.