The amazing properties of rubber and the amazing story of its invention
What do you feel when the goal of your life, which you devoted the last 15 years to, has been achieved? What’s next? The life story of Charles Goodyear, the inventor of rubber, who was born on this day, is just as unusual as the physical properties of rubber itself. Why do most bodies expand when heated and tires, on the contrary, shrink
Since time immemorial, Indians used goods made from the sap of the rubber tree: played with natural rubber balls, covered boat bottoms, made shoes out of it. Europeans, along with smoking, potatoes and chocolate, took over rubber from the Indians as well. Erasers were made of this material. Later, a Scottish chemist named Charles Macintosh while conducting a regular experiment smeared his sleeve jacket with a solution of rubber in turpentine and after a while noticed that the sleeve does not get wet. He patented and began producing waterproof coats - macintoshes.
Natural rubber is one of the simplest natural polymers. It consists of long chain-molecules which consist of repeating blocks:
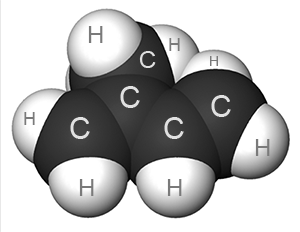
There may be tens of thousands of such blocks in one molecule.
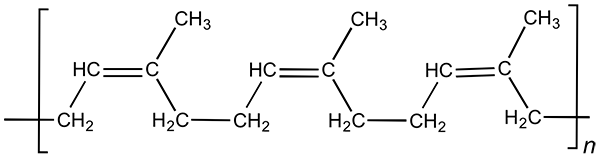
Such a structure has its drawbacks - natural rubber poorly keeps shape. Separate unbounded chain-molecules easily slip over each other, and the material ‘flows’. Because of this natural rubber becomes tacky and soft in warm weather.
The amazing story of the invention
In the first half of the 19th century many people’s minds were busy trying to get rid of this unpleasant property of natural rubber, while keeping all its advantages. As they used to say: to “cure” natural rubber. The American inventor Charles Goodyear also tackled this problem.

And tackled it with passion. Working at home in the kitchen, by trial and error he mixed natural rubber with all that was at hand: coal, sugar, salt, pepper, oil, ink, in the hope that some substance binds natural rubber. His path was thorny. On the way were false hopes, disappointments, partner bankruptcy, and family hunger. But in the end, Charles found a way to “cure” natural rubber. One day he accidentally dropped natural rubber mixed with sulfur into the fireplace. The burnt piece became exactly the way Charles Goodyear wanted to see it.
Despite all of this Goodyear knew little about chemistry. Even having found a way to produce rubber, he did not understand its principles. The second part of his life Charles spent trying to convince the world of the usefulness of his invention. Unfortunately, his sad story does not become happy from now on. Even finding a way to do what he wanted most of his life, Charles was unable to make money on it. He spent all his money on exhibitions, patent disputes, and died in debt.
The amazing properties of rubber
In addition to the human drama, this story is also interesting about what is happening within rubber on a molecular level. Remember, we discussed that the long chain-molecules in rubber easily slip over each other, and this is the cause of its unpleasant properties? So what if these chain-molecules are glued together in some places? This is the process discovered by Goodyear. Sulfur atoms form a “bridge” between the long molecules of rubber, gluing them together and not allowing them to slip.
And how are the remarkable properties of rubber to expand and shrink back explained? What makes it such a wonderful material for slingshots? To understand this we must look at the structure of the molecules of rubber. Here I’ll give the word to Richard Feynman, it is hardly possible to explain it more evidently and clearly – he is definitely a genius!
See also

CASE STUDY - 8th Grade students at St Timothy's Catholic School use MEL Chemistry to enhance their science lessons
Saint Timothy Catholic School in Mesa is committed to promoting academic excellence in each child it looks after. They encourage self-discipline, self-respect, and respect for others. They understand the importance of engaging students in a comprehensive and relevant curriculum. As a result, the middle school science teacher from St. Timothy Catholic School is using MEL Chemistry subscriptions to enhance and expand their range of learning activities.

CASE STUDY - MEL Chemistry allowing pupils to reach their full potential
The Empower Learning Center is the Alternative Learning Program (ALP) within the Hinckley-Finlayson School District. They offer non-traditional education options for students ages 16-21 in their daytime program, night school for traditional high school students who need to make up credits, and night school for adults 18 and older who would like to complete their diploma or equivalency.
The school was seeking engaging, hands-on chemistry kits to make their science classes more interactive, and to help their students understand key science concepts and achieve their full potential in chemistry.

CASE STUDY - MEL Chemistry at Lund International School, Sweden
Emma Taylor, a science teacher at Lund International School (Sweden), has chosen MEL Chemistry sets as the best option for her students’ science classes. In Lund International School, all programmes are taught in English, and having chemistry sets in English are a great asset to accompany science classes.
Here, Emma shares her experience of how MEL Chemistry sets improved her students’ comprehension and understanding of science concepts.