Reveal fingerprints using super glue
This day 102 years ago, the first fingerprint record was taken in USA. Read how to use super glue to reveal latent fingerprints at home. And why superglue sticks to your fingers so much.
We all know that we need to spend more time with our children. It is like with the gym: you know how good working out is for your health but there are always many excuses to postpone it. Let’s use the part of our brain that “knows” to eliminate all possible excuses for the part that “feels”. Do not postpone it! Make an experiment with your children this weekend - reveal latent fingerprints at home using superglue like FBA agents do it.
- It is fun
- Your children will learn some science
- It will not take more than 30 minutes
- You have all you need at home
How does superglue works?
What makes superglue work so well with your fingers and how can you reveal latent fingerprints using it?
The superglue principle is different from most other adhesives. It does not dry out. Superglue consists of small molecules that could join in long chains - polymers. When it happens, superglue sets. To start this process some seed molecules are needed. It could be traces of water on your fingers. This polymerization process is very fast. Super glue contacts with a thin layer of water molecules on your fingers - bang! - long strong chains that bind your fingers are formed.
Why does it happen? To understand this, let’s look at superglue from a chemical point of view. Superglue is a liquid consisting mainly of molecules with a difficult name “methyl-2-cyanoacrylat”.
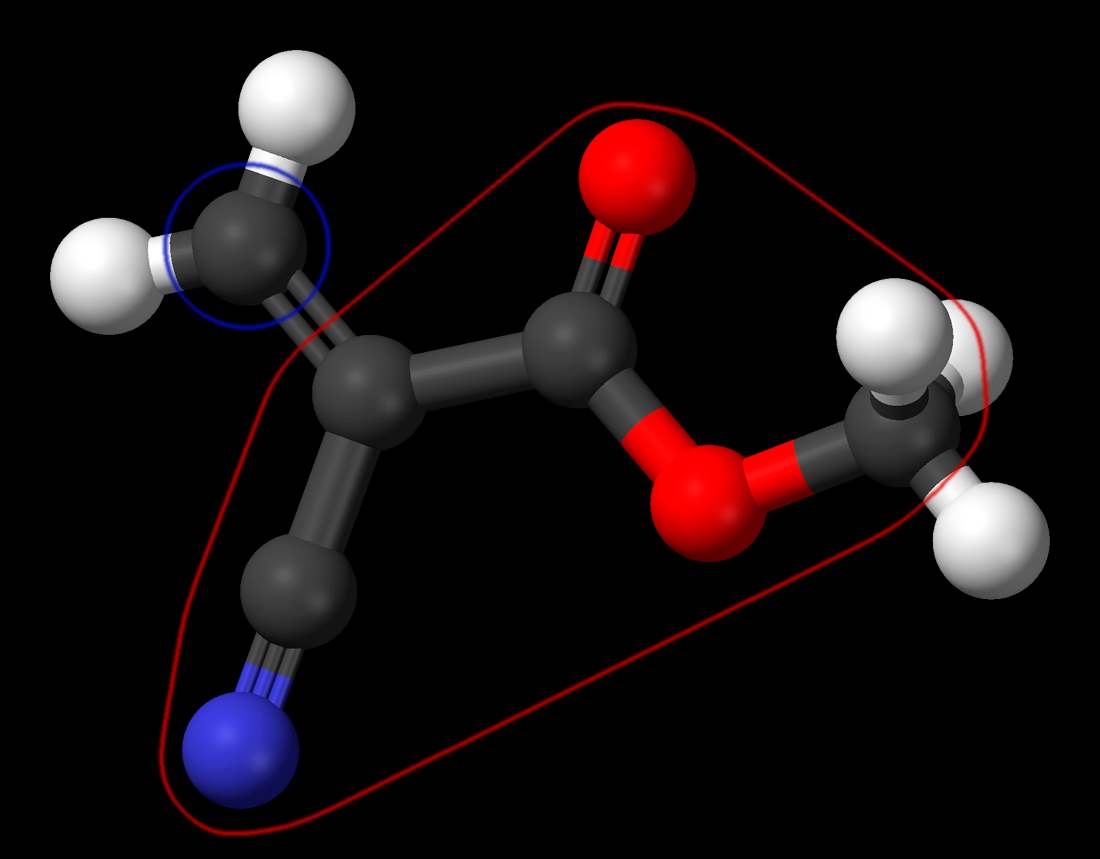
This picture of methyl-2-cyanoacrylat molecule is beautiful but almost useless for us. It does not explain the behavior of this molecule. Moreover it misleads us. However, I do not have a better picture for you yet. So let’s use what we have.
Here is what happens with this molecule step by step:
- Atoms marked by a red circle pull electrons from the carbon atom market with a blue circle. Now this carbon atom has an electron deficit on one of its sides. This means it would be glad to receive an electron pair from someone else who could share it. A water molecule is a good source for such an electron pair.
- After this, the carbon atom has received additional electrons, entire structure of the electron orbitals in the molecule is changed.
- Now another carbon atom has excess of electrons and can share them.
- Since it can share electrons, it can connect to another molecule in the same way as the water molecule did.
- Electron distribution in this new molecule would be changed as well and now it could connect to the third molecule.
- And so on. This chain will grow molecule-by-molecule. Each molecule added to the tail is changed so that it can become a new tail.
A small amount of water is enough to seed this process. Superglue sets.
Fingerprints
Now about out experiment. Superglue can be used to develop fingerprints. Even FBI specialists use this method. Here is what you need:
- a box
- a smooth surface with a fingerprint on it (a CD disk would work perfectly)
- a glass with warm water (to increase air humidity)
- a tray with super glue (you can make one using aluminum foil)
We put all that in our box, close it and wait for 15 minutes. Superglue evaporates and its fumes condense on the fingerprints. After the superglue is condensed it immediately polymerises and we start to see the fingerprints as a white picture. Super glue was even used by hackers to crack iPhone 5S fingerprint scanner.
WARNING! Superglue sticks to your fingers and is difficult to remove. Be careful!
WARNING! Superglue fumes are moderately toxic. It is better to perform this experiment outside or in a well-ventilated room.
There is a similar method of revealing latent fingerprints using iodine. Note that you need solid iodine not a solution. Iodine fumes adhere to oils and other residues of our fingerprints. Iodine is brown which makes fingerprints visible.
That’s it! Now you do not have any excuse not to perform an experiment with your children this weekend.
Subscribe to our Twitter not to miss our next video explaining hydrogen burning process!
See also

CASE STUDY - 8th Grade students at St Timothy's Catholic School use MEL Chemistry to enhance their science lessons
Saint Timothy Catholic School in Mesa is committed to promoting academic excellence in each child it looks after. They encourage self-discipline, self-respect, and respect for others. They understand the importance of engaging students in a comprehensive and relevant curriculum. As a result, the middle school science teacher from St. Timothy Catholic School is using MEL Chemistry subscriptions to enhance and expand their range of learning activities.

CASE STUDY - MEL Chemistry allowing pupils to reach their full potential
The Empower Learning Center is the Alternative Learning Program (ALP) within the Hinckley-Finlayson School District. They offer non-traditional education options for students ages 16-21 in their daytime program, night school for traditional high school students who need to make up credits, and night school for adults 18 and older who would like to complete their diploma or equivalency.
The school was seeking engaging, hands-on chemistry kits to make their science classes more interactive, and to help their students understand key science concepts and achieve their full potential in chemistry.

CASE STUDY - MEL Chemistry at Lund International School, Sweden
Emma Taylor, a science teacher at Lund International School (Sweden), has chosen MEL Chemistry sets as the best option for her students’ science classes. In Lund International School, all programmes are taught in English, and having chemistry sets in English are a great asset to accompany science classes.
Here, Emma shares her experience of how MEL Chemistry sets improved her students’ comprehension and understanding of science concepts.