Does methane on Mars indicate the presence of life?
Is methane on the Mars the evidence of life? Science journal published an article containing information about huge amounts of methane in the atmosphere of Mars two days ago. Moreover, scientists claim that methane does not only exist but also being produced on Mars. Can this discovery suggest existence of life on a Mars?

Let’s consider the news in a more detailed way. So the Curiosity found 0.7 ppb (parts per billion) methane on the average in the air of Mars. Additionally, scientists observed elevated levels of methane - 10 times more - implying that Mars is episodically producing methane from unknown source. Here we come up with 3 questions:
- What is methane and why it is important for life on the planet?
- How is new methane produced?
- Can it help create or support life on the planet?
Let’s consider these 3 questions one by one.
1. What is methane and why is it important for life on the planet?
Methane is a gaseous substance with the formulae CH4 which means that the molecule contains one carbon and four hydrogen atoms. It is common knowledge that carbon is the key component of life on Earth. It is the reason to search for carbon-containing substances in space and on other planets. Moreover, methane is a greenhouse gas. Greenhouse gases can absorb infrared irradiation emitted by warm objects on the surface of the planet and stop this irradiation from going out to space. This is a way to support a constant temperature on the planet surface.
2. What are possible ways of producing methane?
Methane occurs naturally in geological processes (that’s why it is called natural gas). Also we know that methane can be produced by agriculture – and it’s the main point to claim that methane can be evidence of life on any planet.
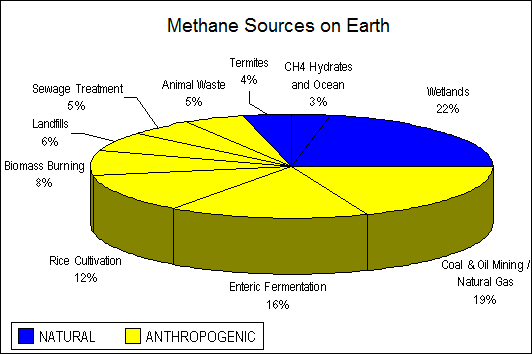
How is methane produced on Mars? According to a paper published in Science on December 16, 2014 we can find several possible sources of methane on Mars:
- Biological - methanogenic organisms
- Geological production during changes in the structure of minerals caused by the inclusion of water inside the crystal structure (this process is called serpentization). On Mars the mineral olivine can undergo seprentization.
- Ultraviolet degradation of metheoritically-delivered organics.
- Release from subsurface substances called clathrates
- Gas absorbed by the mineral regolith
- Erosion of basalt with methane inclusions
- Geothermal production (similar to processes on Earth)
3. Can it help create or support life on the planet?
Here we see many options and only one of them involves existence of life on a Mars. So obviously we cannot be sure or even hope that life exists on the Red Planet. However, the increase of methane in the atmosphere of Mars can be good for Mars colonization: if the greenhouse effect will occur on Mars, we can hope to use it to increase Mars’ temperature to make living condition for colonists closer to Earth conditions.
Subscribe to our Twitter not to miss our next video explaining hydrogen burning process!
See also

CASE STUDY - 8th Grade students at St Timothy's Catholic School use MEL Chemistry to enhance their science lessons
Saint Timothy Catholic School in Mesa is committed to promoting academic excellence in each child it looks after. They encourage self-discipline, self-respect, and respect for others. They understand the importance of engaging students in a comprehensive and relevant curriculum. As a result, the middle school science teacher from St. Timothy Catholic School is using MEL Chemistry subscriptions to enhance and expand their range of learning activities.

CASE STUDY - MEL Chemistry allowing pupils to reach their full potential
The Empower Learning Center is the Alternative Learning Program (ALP) within the Hinckley-Finlayson School District. They offer non-traditional education options for students ages 16-21 in their daytime program, night school for traditional high school students who need to make up credits, and night school for adults 18 and older who would like to complete their diploma or equivalency.
The school was seeking engaging, hands-on chemistry kits to make their science classes more interactive, and to help their students understand key science concepts and achieve their full potential in chemistry.

CASE STUDY - MEL Chemistry at Lund International School, Sweden
Emma Taylor, a science teacher at Lund International School (Sweden), has chosen MEL Chemistry sets as the best option for her students’ science classes. In Lund International School, all programmes are taught in English, and having chemistry sets in English are a great asset to accompany science classes.
Here, Emma shares her experience of how MEL Chemistry sets improved her students’ comprehension and understanding of science concepts.