20 years ago, scientists created the element 111 - Roentgenium
20 years ago, scientists created the element #111. Who would care about an element that lived less than one second? Read how our life is obligated to another element whose life is billions of times shorter.
Today, we celebrate 20 years as a team of scientists led by German physicist Sigurd Hofmann who created a new element #111 called Roentgenium. We say created and not discovered because this element did not exist on our planet before. How can you create a new element? You take two lighter nuclei (in that case, it was element #83 and element #28), accelerate one of them and smash it into the second. If you choose the right speed, which is fast enough to break the repulsion barrier, but not too fast to blow up all the protons and neutrons you could get a new element.
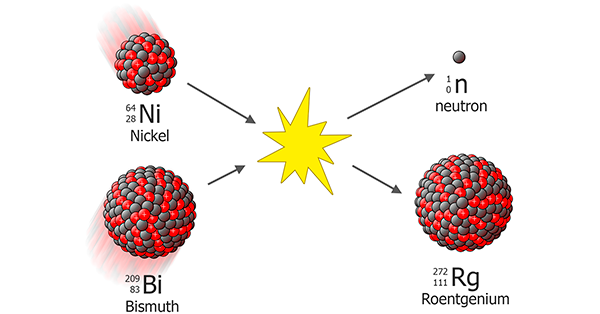
That is what they did in the GSI Helmholtz Centre for Heavy Ion Research near Darmstadt, Germany. Just three atoms of the new element were produced which decay in less than one hundredth of a second.
When you create a new element, you have to wait until independent experts could reproduce your experiment before the new element is officially accepted by IUPAC and a name is given. It took ten years for Roentgenium to be officially recognized. Before that, the element was known under a temporary name “Unununium”. Which is based on its number 111: un (one) un (one) un (one) ium. There are five elements now that do not have their names yet and are known under their temporary names:
- Ununtrium (#113)
- Ununpentium (#115)
- Ununseptium (#117)
- Ununoctium (#118)
- Ununennium (#119)
Almost all of them were first created by the Joint Institute for Nuclear Research in Dubna, Russia.
Why should anyone care about an element that decays much faster than the time it takes you to blink? I do not know how this particular element could be useful but let me show you one example of how the existence of our life is obligated to another element wherein its life is even shorter, billions of times shorter.
The story will be about element #4 - Beryllium. Most of beryllium existing has 4 protons and 5 neutrons. Such beryllium is stable and does not decay. However, there is another form of beryllium, which has 4 protons and only 4 neutrons. Such beryllium-8 is very unstable. The half-life is less than one billionth of a second. Nevertheless, if it would not exist, the entire world as we know it would be very different.
As you know, only the lightest elements hydrogen and helium were produced during the big bang. All the other elements are produced in stars. Right now our Sun is producing helium by fusing hydrogen. When the star starts to run out of hydrogen to fuse, it starts to collapse. The temperature increases to a hundred million degrees to create conditions for helium to fuse. The next stable element that can be formed from helium is carbon-12. It requires three helium-4 to fuse. The chance that three helium nuclei meet together in one place is very low. If two helium nuclei collide they form beryllium-8 which, as was said, is very unstable and lives less than one billionth of a second but this time it is big enough that there is a chance that the third helium will collide to form stable carbon-12.
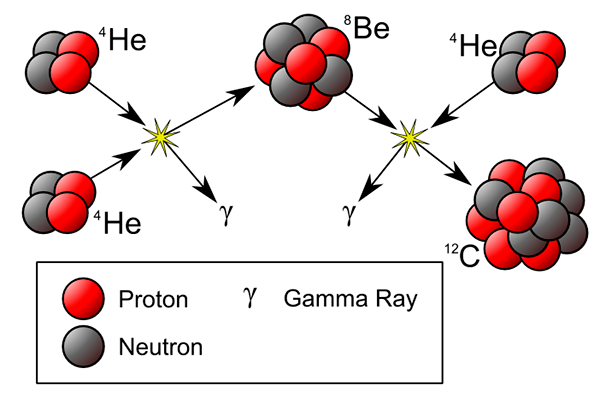
It is a short story of how the existence of carbon and all heavier elements that form life on our Earth and the Earth itself depend on one element beryllium-8 where the half-life time is less than 0.0000000000000001 seconds. Does it answer the question of theelement #111 Roentgenium could have any influence on our life? I do not think so. However, things are often not as simple as they seem when we think if some knowledge is useful or not. Maybe now you will be more interested in watching a short video about the creation of Roentgenium that happened 20 years ago.
Subscribe to our Twitter not to miss our next video explaining hydrogen burning process!
See also

CASE STUDY - 8th Grade students at St Timothy's Catholic School use MEL Chemistry to enhance their science lessons
Saint Timothy Catholic School in Mesa is committed to promoting academic excellence in each child it looks after. They encourage self-discipline, self-respect, and respect for others. They understand the importance of engaging students in a comprehensive and relevant curriculum. As a result, the middle school science teacher from St. Timothy Catholic School is using MEL Chemistry subscriptions to enhance and expand their range of learning activities.

CASE STUDY - MEL Chemistry allowing pupils to reach their full potential
The Empower Learning Center is the Alternative Learning Program (ALP) within the Hinckley-Finlayson School District. They offer non-traditional education options for students ages 16-21 in their daytime program, night school for traditional high school students who need to make up credits, and night school for adults 18 and older who would like to complete their diploma or equivalency.
The school was seeking engaging, hands-on chemistry kits to make their science classes more interactive, and to help their students understand key science concepts and achieve their full potential in chemistry.

CASE STUDY - MEL Chemistry at Lund International School, Sweden
Emma Taylor, a science teacher at Lund International School (Sweden), has chosen MEL Chemistry sets as the best option for her students’ science classes. In Lund International School, all programmes are taught in English, and having chemistry sets in English are a great asset to accompany science classes.
Here, Emma shares her experience of how MEL Chemistry sets improved her students’ comprehension and understanding of science concepts.